DSS: Redefining Biotechnology & Life Science in India
Clinical Diagnostics, Abbott Molecular
Thermobrite
Abbott Molecular ThermoBrite® System offers an easy, safe, system for in-situ hybridization procedures.
Clinical Diagnostics, Abbott Molecular
AneuVysion
AneuVysion, which utilizes patented fluorescence in situ hybridization (FISH) technology applied to uncultured amniocytes, provides detection of trisomies 13, 18, and 21 (Down syndrome) and sex chromosome aneusomies in as little as 24 hours.
Clinical Diagnostics, Abbott Molecular
Genetic Probes
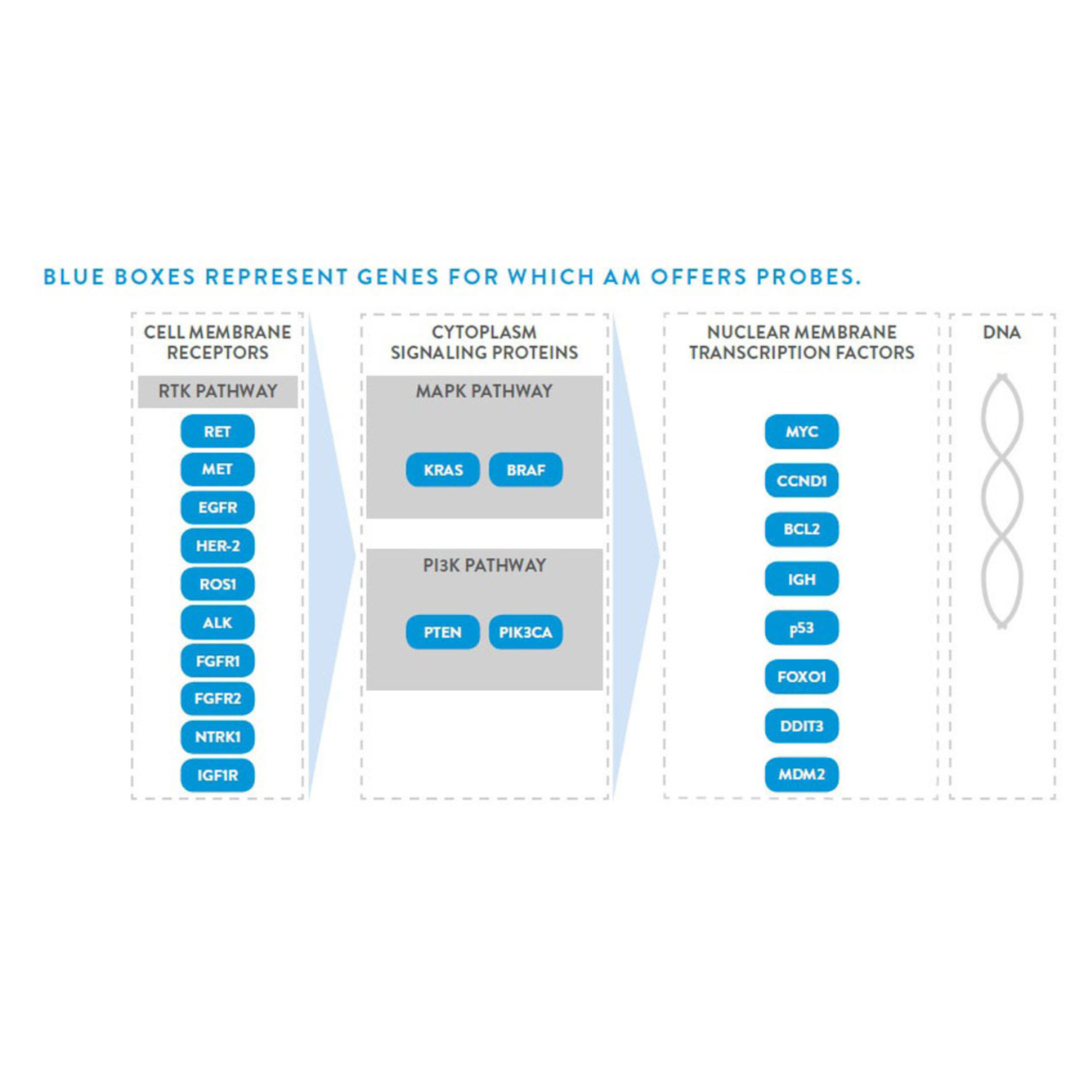
Genetic Probes are used for detection of chromosomal anomalies at prenatal, postnatal and preimplantation stage. These probes enable rapid, sensitive and specific dection, identification & characterization of chromosome anomalies.
Clinical Diagnostics, Abbott Molecular
Hematology Probes
Abbott Molecular offers a wide range of Vysis DNA Fluorescence in situ Hybridization (FISH) products for the effective and rapid identification of genetic aberrations associated with hematopoietic disorders.
Clinical Diagnostics, Abbott Molecular
PathVysion
The PathVysion HER-2 DNA Probe Kit II (PathVysion Kit II) is designed to detect amplification of the HER-2/neu gene via fluorescence in situ hybridization (FISH) in formalin-fixed, paraffin-embedded human breast and gastric cancer tissue specimens.
Clinical Diagnostics, Abbott Molecular
Solid Tumor Probes
The detection of genetic aberrations in solid tumors with DNA Fluorescence in situ Hybridization (FISH) probe technology is a powerful means to diagnose and more efficiently treat a wide range of cancers. Vysis offers a comprehensive line of direct labeled DNA probes for solid tumor assessment.
Clinical Diagnostics, Abbott Molecular
UroVysion
The UroVysion® Bladder Cancer Kit (UroVysion Kit) is FDA approved and designed to detect aneuploidy for chromosomes 3, 7, 17, and loss of the 9p21 locus via fluorescence in situ hybridization (FISH) in urine specimens from persons with hematuria suspected of having bladder cancer.
Clinical Diagnostics, Abbott Molecular
VP2000
VP2000 is a consolidated workstation for
automated front-end FISH* processing
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HPV Assay
Abbott RealTime High Risk HPV provides clinically proven and validated results allowing for improved and effi cient patient management in cervical cancer screening.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime CMV Assay
The CMV PCR Kit constitutes a ready-to use system for the detection of CMV DNA using polymerase chain reaction (PCR).
Clinical Diagnostics, Abbott Molecular
Abbott RealTime CT/NG Assay
The Abbott RealTime CT/NG assay is an in vitro polymerase chain reaction (PCR) assay for the direct, qualitative detection of the plasmid DNA for Chlamydia trachomatis and the genomic DNA of Neisseria gonorrhoeae in female endocervical or vaginal swab specimens, male urethral swab specimens, or male and female urine specimens.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HCV-GTII Assay
The Abbott RealTime HCV Genotype II is an in vitro reverse transcription-polymerase chain reaction (RT-PCR) assay for determining the genotype(s) of hepatitis C virus (HCV) in plasma and serum from HCV-infected individuals.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HIV-1 Qualitative Assay
The Abbott RealTime HIV-1 Qualitative assay aids laboratories and physicians in diagnosing HIV-1 infections in pediatric and adult patients, providing usage of innovative DBS sampling and reliable detection of all HIV-1 groups and subtypes.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HCV Assay
The Abbott RealTime HCV assay is an in vitro reverse transcription-polymerase chain reaction (RT-PCR) assay for the quantitation of hepatitis C viral ribonucleic acid (HCV RNA) in human serum and plasma from HCV-infected individuals.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HBV Assay
The Abbott RealTime HBV is an in vitro polymerase chain reaction (PCR) assay for the quantitation of Hepatitis B Virus (HBV) DNA in human plasma or serum from HBV-infected individuals.
Clinical Diagnostics, Abbott Molecular
Abbott RealTime HIV-1 Assay
The Abbott RealTime HIV-1 assay is an in vitro reverse transcription-polymerase chain reaction (RT-PCR) assay for the quantitation of Human Immunodeficiency Virus type 1 (HIV-1) on the automated m2000 System in human plasma from HIV-1 infected individuals over the range of 40 to 10,000,000 copies/mL. The Abbott RealTime HIV-1 assay is intended for use in conjunction with clinical presentation and other laboratory markers for disease prognosis and for use as an aid in assessing viral response to antiretroviral treatment as measured by changes in plasma HIV-1 RNA levels.
Testimonials & Reviews