DSS: Redefining Biotechnology & Life Science in India
- About Us
- Products & Services
PRODUCTS & SERVICES
-
Kits Reagents & Consumables
- Cytogenetics
- Dyes
- Fluorescence In Situ Hybridization (FISH)
- High-Performance Liquid Chromatography (HPLC)
- Histology
- Immuno Histo Chemistry (IHC)
- Molecular Pathology & Diagnostics
- Multiplex Ligation-Dependent Probe Amplification (MLPA)
- Nucleic Acid Extraction
- PharmDx
- Real Time PCR
- Sequencing
- Special Stains
-
Instruments
- Biosafety
- Cameras
- Cell Culture monitoring
- CO2 Incubator
- Cytogenetics Systems
- Digital Slide Scanners
- Electrophysiology Systems
- Endoscopy System
- Fluorescence In Situ Hybridization (FISH)
- H&E
- Immuno Histo Chemistry (IHC)
- Microfluidics
- Microscopes
- Molecular Pathology & Diagnostics
- Multichannel Imaging Systems
- Real Time PCR
- Special Stains
- Software
- Accessories
- Advanced Material
-
Kits Reagents & Consumables
- Applications & Specialities
All Applications & Specialities
- Brands
- Contact Us
-

-
 0
0
- ☰
- About Us
- Products & Services
-
Kits Reagents & Consumables
- Cytogenetics
- Dyes
- Fluorescence In Situ Hybridization (FISH)
- High-Performance Liquid Chromatography (HPLC)
- Histology
- Immuno Histo Chemistry (IHC)
- Molecular Pathology & Diagnostics
- Multiplex Ligation-Dependent Probe Amplification (MLPA)
- Nucleic Acid Extraction
- PharmDx
- Real Time PCR
- Sequencing
- Special Stains
-
Instruments
- Biosafety
- Cameras
- Cell Culture monitoring
- CO2 Incubator
- Cytogenetics Systems
- Digital Slide Scanners
- Electrophysiology Systems
- Endoscopy System
- Fluorescence In Situ Hybridization (FISH)
- H&E
- Immuno Histo Chemistry (IHC)
- Microfluidics
- Microscopes
- Molecular Pathology & Diagnostics
- Multichannel Imaging Systems
- Real Time PCR
- Special Stains
- Software
- Accessories
- Advanced Material
-
Kits Reagents & Consumables
- Applications & Specialities
- Brands
- Brand - Life Sciences
- 3i
- ABBERIOR INSTRUMENTS
- Abbott Molecular
- ADS Biotec
- APPLIED SPECTRAL IMAGING
- BioAir Tecnilabo
- DAKO (AGILENT)
- Eden Tech
- Elveflow
- ENTROGEN
- EUROCLONE
- EVIDENT
- Genea
- Genial Helix
- Hamamatsu Photonics
- Invivoscribe
- MASTER DIAGNOSTICA
- MBF BIOSCIENCE
- Medical Tek Co. Ltd
- MILESTONE MED SRL
- Molecular Machines & Industries
- MRC HOLLAND
- NeoDx
- Onward Assist
- PHOTOMETRICS
- Profound
- SCIENTIFICA
- µCyte
- Seqlo
- Brand - Industrial
- Brand - Life Sciences
- News & Events
- Career
- Contact Us
- Testimonial
- Blog
- R&D
- CSR


Home > Products & Services > Kits Reagents & Consumables > Fluorescence In Situ Hybridization (FISH) > Oncology FISH Probes > UroVysion
UroVysion
The UroVysion® Bladder Cancer Kit (UroVysion Kit) is FDA approved and designed to detect aneuploidy for chromosomes 3, 7, 17, and loss of the 9p21 locus via fluorescence in situ hybridization (FISH) in urine specimens from persons with hematuria suspected of having bladder cancer. Results from the UroVysion Kit are intended for use, in conjunction with and not in lieu of current standard diagnostic procedures, as an aid for initial diagnosis of bladder carcinoma in patients with hematuria and subsequent monitoring for tumor recurrence in patients previously diagnosed with bladder cancer.
The UroVysion Bladder Cancer Kit probes are directly labeled with one of the Vysis fluorophores; SpectrumRed, SpectrumGreen, SpectrumAqua or SpectrumGold. The UroVysion Bladder Cancer Kit consists of three alpha-satellite repeat sequence probes; CEP 3 SpectrumRed, CEP 7 SpectrumGreen, and CEP 17 SpectrumAqua that hybridize to the centromere regions of chromosomes 3, 7, and 17, respectively. In addition, a unique sequence probe, LSI p16 (9p21) SpectrumGold, is included that hybridizes to the p16 gene at 9p21. This probe set is premixed in Hybridization Buffer.
-
Add to WishlistAdd to Wishlist
About
Abbott Molecular Laboratories Healthcare Equipments in India
Abbott Molecular Diagnostics, a world leader in the field of DNA based molecular diagnostics, has its solutions for real-time PCR (Infectious Diseases) and FISH (Vysis) used to conduct sophisticated analysis of patient DNA and RNA. Using Abbott Molecular Diagnostics products, physicians can access critical information by early detection of pathogens and subtle changes in patients' genes, with the following benefits:
Early Diagnosis
Selection Appropriate Therapies
Monitoring Disease Progression
More Products
Other Cytogenetic Culture Media Products
Other Cytogenetic Culture Media Products are also available from Euroclone
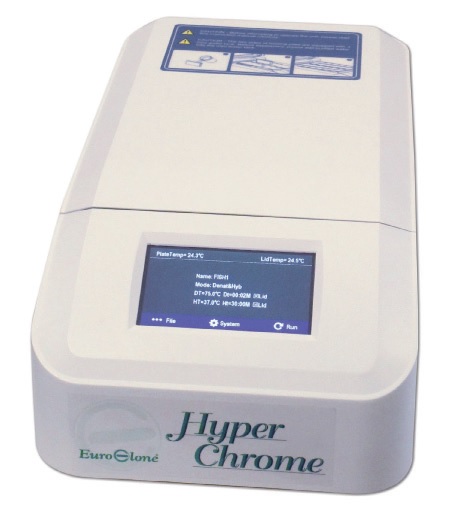
Hyperchrome
HyperChrome is the perfect Instrument for denaturation & hybridization procedures during FISH experiments.
Prenatal Analysis Cytogenetic Culture Media Reagents
Prenatal Analysis Cytogenetic Culture Media Reagents include Chorionic Villi and In Situ
Postnatal Analysis Cytogenetic Culture Media Reagents
Postnatal cytogenetic analysis refers to the karyotyping of samples derived from a variety of tissues:…
Testimonials & Reviews

Dr. Chhaya Chande, Professor & HOD, Microbiology
GGMCJJ Hospitals, Mumbai
“Ms. Megha Dhumal (Assistant Manager- Application) has done a satisfactory demonstration of the running of the Abbott Sample preparation machine model m2000sp and the Abbott RT-PCR machine model m2000rt. We appreciate the effort made by the DSS team under these difficult conditions to help our lab to carry out the imperative Covid-19 tests.”

Dr Sunil K Arora, Professor, Deptt of Immunopathology
PGIMER, Chandigarh
“We are using Confocal Microscope and one Fluorescence Microscope. Both are working fine. The after sales services by DSS have been excellent for functioning & upkeep of the microscopes. The applications support by experts from DSS is very useful. Keep it up!”

Dr Pramod Kumar Bajaj
MD, Spermprocessor Pvt Ltd
“Really excited to see the DSS Pathology solutions exhibition booth at APCON 2019 along with Magnus. We think all the upcoming technology had been displayed along with your efforts to make it Indigenous (Made in India) is highly appreciated. Wish you all the best. Keep it up!”

Dr. Sreejesh S, Associate Professor, Dept of Hematology
PGIMER, Chandigarh
“My experience with DSS so far has been very good till now. We are getting good support in both purchase as well as in troubleshooting. Very good experience with Mr Arun, Mr Manoj, Mr Mahesh and all others from the DSS team.”
Dr Sudha S Murthy, Department of Pathology and Laboratory Medicine
BIACH & RI, Hyderabad
“I am happy with DSS and associated with 19 years and use Dako antibody. Happy with Supply but need improvement.”

Dr S Radhika MD, PhD
Professor, Deptt. Of Cytology & Gynaec Pathology, PGIMER, Chandigarh
“PGI Cytology Dept. has had a long association with DSS- Olympus Microscopy Division. They have provided excellent services- after sales service. The product is also of very good quality. We have had no problems with their products and services are of very good quality.”

Dr Nuzhat Husain
RMLIMS, Lucknow
“Have been using Dako Reagants and Dako antibodies for a while. Services and products have been good and timely.”

Dr Minu Singh
Assistant Professor, PGIMER, Chandigarh
“MRC Holland MLPA products provided by DSS are of good quality, have never faced any quality issues with their product or shipping condition. They provide prompt response upon any query.”

Mr. Krishnani Professor, SGPGI, Lucknow
“My experience with DSS so far has been excellent for the last 30 years- sales and service experience. Microscope products are very useful and sturdy with high precision.”