DSS: Redefining Biotechnology & Life Science in India
- About Us
- Products & Services
PRODUCTS & SERVICES
- Applications & Specialities
All Applications & Specialities
- Brands
- Contact Us
-

-
 0
0
- ☰
- About Us
- Products & Services
-
Kits Reagents & Consumables
- Cytogenetics
- Dyes
- Fluorescence In Situ Hybridization (FISH)
- High-Performance Liquid Chromatography (HPLC)
- Histology
- Immuno Histo Chemistry (IHC)
- IVF Consumables
- Molecular Pathology & Diagnostics
- Multiplex Ligation-Dependent Probe Amplification (MLPA)
- Nucleic Acid Extraction
- PharmDx
- Real Time PCR
- Special Stains
- Instruments
- Software
- Accessories
- Advanced Material
- Therapies
-
Kits Reagents & Consumables
- Applications & Specialities
- Brands
- Brand - Life Sciences
- 3i
- ABBERIOR INSTRUMENTS
- Abbott Molecular
- ADS Biotec
- APPLIED SPECTRAL IMAGING
- BioAir Tecnilabo
- DAKO (AGILENT)
- Eden Tech
- Elveflow
- ENTROGEN
- EUROCLONE
- EVIDENT
- Genea
- Hamamatsu Photonics
- Invivoscribe
- MASTER DIAGNOSTICA
- MBF BIOSCIENCE
- MBST
- Medical Tek Co. Ltd
- MILESTONE MED SRL
- Molecular Machines & Industries
- MRC HOLLAND
- NeoDx
- Onward Assist
- Profound
- SCIENTIFICA
- SpaceGen
- Seqlo
- µCyte
- Brand - Industrial
- Brand - Life Sciences
- News & Events
- Career
- Contact Us
- Testimonial
- Blogs
- R&D
- CSR
- Press Release

Cervical Cancer: Awareness, Early Diagnosis, and Prevention
BY Aditi Sahu, Application Specialist 12th January 2026
A January Focus on Cervical Cancer: Early Detection Saves Lives
January is dedicated to raising awareness about cervical cancer, its causes, and prevention strategies. The month promotes women’s health, cervical cancer awareness and diagnosis, and preventive care. Regular screening and immunization can often prevent cervical cancer, but many instances go undetected because of ignorance. Raising awareness about cervical cancer is vital for early diagnosis because the disease frequently exhibits no symptoms, making routine screening tests necessary even when a person feels healthy and shows no symptoms.
What is HPV, and how is it transmitted?
Human papillomavirus (HPV) is a virus that causes sexually transmitted infections. A large family of over 150 related DNA viruses, responsible for infecting skin and mucous membranes to cause STDs. Persistent infections can result in a number of health issues, even if the body can occasionally eradicate the virus on its own.
However, certain strains of the virus can lead to conditions such as genital warts and cancer. HPV 16 and 18 are the major cancer-causing strains.
Sexual activity, including oral, anal, and vaginal contact, may transmit HPV. Even if there are no obvious signs of infection, the virus remains capable of spreading from person to person
In certain circumstances, the body can eliminate HPV on its own without harm. However, if the infection continues, cells may undergo aberrant abnormalities that may ultimately result in cancer.
HPV Induced Oncogenesis
High-risk HPV causes cancer by infecting skin cells, integrating its DNA, and generating proteins (E6/E7) that prevent cell death (apoptosis), encourage unchecked growth, interfere with regular cell cycles, and cause DNA damage accumulation. Over years or decades, the majority of infections are eradicated by the immune system, but persistent ones can cause lesions (dysplasia) that can worsen.
Elimination:
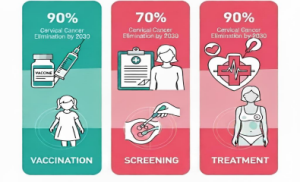
To eliminate cervical cancer, all countries should aim to reduce and maintain the incidence rate below 4 per 100,000 women. Achieving this depends on three key strategies.
Vaccination: Ensure that 90% of girls are fully vaccinated with the HPV vaccine by age15.
Screening: Achieve 70% coverage of women screened using a high performance test by age 35 and again by age 45.
Treatment: Treat 90% of women with pre-cancerous lesions. Properly manage 90 % of women with invasive cervical cancer.
Reference – https://www.who.int/initiatives/cervical-cancer-elimination-initiative
90-70-90 Target
Worldwide and Nationwide Statistics and Government Measures
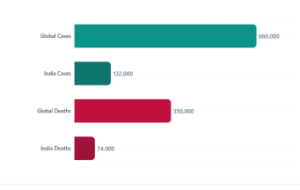
With over 660 000 new cases and 350 000 deaths in 2022, cervical cancer is the fourth most frequent malignancy in women worldwide.
Nations with lower or middle incomes have the highest rates of cervical cancer incidence and mortality. This illustrates significant disparities caused by social and economic factors, a lack of access to the national HPV vaccine, and cervical screening and treatment services.
According to current estimates, India accounts for over one-third of all cervical cancer deaths worldwide, with 132,000 new cases diagnosed and 74,000 deaths every year.
Over 10.18 crore women nationwide who are 30 years of age or older have been screened for cervical cancer, marking a significant milestone in women’s health for the Ministry of Health & Family Welfare. This accomplishment is a component of the population-based program that the National Health Mission (NHM) is implementing through Ayushman Arogya Mandirs (AAMs) for the screening, prevention, and management of non-communicable diseases (NCDs).
The program primarily uses Visual Inspection with Acetic Acid (VIA) at Sub-Health centres and Primary Health Centres under AAM by qualified health professionals to screen women between the ages of 30 and 65. Cases that test positive for VIA are sent to higher centres for additional diagnostic testing.
Reference –
1) https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
2) https://www.mohfw.gov.in/?q=en/pressrelease-279
Diagnosis Techniques:
HPV diagnosis involves a variety of procedures, including visual clinical examinations of genital warts and advanced laboratory testing.
- Cytological tests, such as the Pap smear, detect cellular alterations but not the virus itself.
- Histopathology (biopsies) validates tissue abnormalities when cancer is suspected. PCR, the “gold standard”, and Hybrid Capture assays, which can detect and genotype high-risk viral DNA with great sensitivity.
- Serological testing for antibodies exists; it is generally used in research rather than ordinary clinical practice.
- Molecular approaches are critical for early identification and accurate cervical cancer screening.
HPV Molecular Testing:
Molecular HPV diagnosis is based on the direct detection and identification of viral nucleic acids, which provides significantly greater sensitivity than traditional smears.
The technique involves the following steps:
- Sample collection: Collecting a sample from epithelial cells with a sterile brush or swab
- Extraction: extracting DNA to isolate the viral genome
- PCR: Polymerase Chain Reaction (PCR), which amplifies specific HPV genes, such as the L1 gene, to detectable levels. Variants such as real-time PCR (qPCR) and multiplex PCR enable the quantification and simultaneous detection of numerous strains
- Sequencing/Genotyping: Genotyping is then used to identify high-risk strains, such as HPV-16 and HPV-18, which are the most closely associated with cervical cancer. Finally, the data are examined using fluorescent probes or sequencing, giving clinicians a clear path for patient care.
DSS Imagetech Offers Molecular Testing Kits for HPV diagnosis from Certest Viasure, Abbott m2000 System – Automated HPV Testing & NeoDx Cervsure HPV Detection Kit.
1. Certest HPV Testing Kit (VIASURE High-Risk HPV Real-Time PCR):
This kit is a molecular diagnostic kit that uses real-time PCR to identify HPV DNA. It is a qualitative assay. The test amplifies and detects HPV DNA using Real-Time PCR (Polymerase Chain Reaction) technology. HPV types 16 and 18, which are most closely associated with cervical cancer, are among the 14 high-risk HPV types that it targets. A cervical sample (usually taken by a doctor) is used to extract DNA, which is then amplified and identified using fluorescent probes. The high-risk HPV kinds that it can identify are 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.Additionally, it identifies and flags HPV-16 and HPV-18, which together cause most HPV-related cervical malignancies.
Workflow of HPV Testing with VIASURE Certest Kit –
- Sample Collection – A medical expert will gather cervical specimens (such as cervical swabs in liquid-based cytology medium). Prior to PCR, clinical DNA extraction is necessary.
- DNA Extraction – Purified HPV DNA, if any, is obtained for amplification by extracting DNA from the collected sample using the proper DNA extraction kit.
- Reaction Configuration – Use the supplied rehydration buffer to rehydrate the lyophilized PCR master mix in each well. Fill each well with a determined volume of isolated DNA, usually about 5 µL. Incorporate internal control, negative control, and positive control wells into the same run.
- Real-Time PCR Amplification – Put the plate or reaction strips in a real-time PCR machine. Follow the prescribed heat cycling procedure; each cycle’s fluorescence is measured to track amplification in real time. Target DNA amplification improves fluorescence since the kit uses 5′ nuclease chemistry (TaqMan probe hybridization and cleavage).
HPV Testing Kits from VIASURE Certest
- VIASURE Real-Time PCR Detection Kit for High-Risk Human Papillomavirus recognizes HPV-16 and HPV-18 in particular and detects DNA from 14 high-risk HPV strains
- VIASURE Real-Time PCR Detection Kit for Papillomavirus 16 + 18. Specifically identifies and distinguishes between HPV types 16 and 18
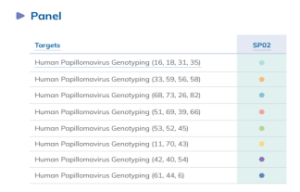
- VIASURE Human Papillomavirus Genotyping Panel (Sexual Health Panel 02): 28 HPV strains are detected using a variety of qPCR assays (RUO, not for diagnosis). Below is the image of the HPV genotyping panel available in Certest. The panel is made up of eight-well strips, each of which represents a distinct test. The panel also includes an endogenous internal control (EIC) (RNase P gene) and an internal control (IC) to monitor the integrity of the sample to keep an eye on the extraction procedure, rule out extraction issues, and/or confirm that the amplification is operating well.
The panel is made up of eight-well strips, each of which represents a distinct test. The panel also includes an endogenous internal control (EIC) (RNase P gene) and an internal control (IC) to monitor the integrity of the sample to keep an eye on the extraction procedure, rule out extraction issues, and/or confirm that the amplification is operating well.
2. Abbott M2000 System – Automated HPV Testing:
The Abbott m2000 System uses the real-time PCR molecular test that detects 14 high-risk HPV genotypes from cervical liquid-based cytology samples. It provides automated extraction and amplification, differentiates HPV-16 and HPV-18, and offers high sensitivity and specificity for HPV screening, aiding cervical cancer prevention programs.
- M2000 sample extraction: The DNA extraction procedure that makes use of the sample Preparation System DNA kit. This procedure includes lysis, micro particle capture, washing, and final elution. DNA is extracted automatically in the instrument thus decreasing manual labour for extraction.
- Internal control: The use of endogenous human beta-globin as an internal control, which is present throughout extraction and testing to guarantee sample validity and check for adequate cell input.
- M2000rt – Real-Time PCR Detection: The m2000RT provides real-time amplification and detection. Real-time thermal cycler used to amplify copies of DNA.
- Assay design: The test design uses real-time PCR technology to target the highly conserved L1 region of the HPV genome. To differentiate between targets, it uses short, single-stranded hybridization probes labelled with several dyes: VIC for HPV 16, NED for HPV 18, and FAM for a group of 12 additional high risk genotypes (31,33,35,39,45,51,52,56,58,59,66 and 68).
- PCR interpretation: A fixed cycle number (CN) limit of 32.00 is specified for all HPV signals because the objective is to detect clinically relevant illness rather than every single virus molecule. In a similar vein, the internal control has a threshold of 35.00 to detect possible false negatives resulting from inadequate cell input or DNA loss. https://www.dssimage.com/product/abbott-realtime-hpv-assay/
The panel is made up of eight-well strips, each of which represents a distinct test. The panel also includes an endogenous internal control (EIC) (RNase P gene) and an internal control (IC) to monitor the integrity of the sample to keep an eye on the extraction procedure, rule out extraction issues, and/or confirm that the amplification is operating well.
3. NeoDx Cervsure HPV Detection Kit:
The test simultaneously amplifies and detects several genetic targets in a single run because it is a multiplex, TaqMan probe-based real-time PCR assay.
Cervsure HPV detection kit includes the following kits –
Cervsure Human Papillomavirus (HPV) Screening Real-Time PCR Kit (14 High Risk) – A multiplex real-time PCR test that uses DNA taken from clinical swab samples to identify and distinguish between HPV-16 and HPV-18 while looking for 12 other high-risk HPV genotypes.

Cervsure Real-Time PCR Kit for Human Papillomavirus (HPV) Screening (18 High Risk) – A multiplex real-time PCR test that looks for 16 high-risk HPV genotypes in DNA from cervical or oral samples and detects HPV-16 and HPV-18.

Cervsure HPV DNA Extraction Kit – This kit is intended to separate and purify HPV DNA from clinical specimens, such as cervical swabs, in order to prepare the sample for PCR testing later on.

Sample Collection Kit for HPV Screening is a sample collection tool and media designed to securely gather and transport vaginal or cervical samples for later extracting HPV DNA.
Since HPV 16 and 18 are the most dangerous forms, the kit usually targets them separately while simultaneously screening for a panel of additional high-risk HPV genotypes.
Target region: The NeoDx HPV detection kits, such as the mPlex HPV High-Risk Screening Kit, typically target the E6 and E7 oncogene regions of the HPV genome.
Internal control: To confirm that the PCR was effective and that DNA extraction was successful, an internal control, typically beta-actin, is added.
Reporting format: A positive result means that HPV DNA from one or more of the test panel’s high-risk genotypes is present. Cervical cancer and precancerous lesions are linked to persistent high-risk HPV infection.
A negative result indicates that no HPV DNA was found in the sample above the test’s detection limit.
Reference – https://www.neodx.in/hpv-high-risk-screening-real-time-pcr
In screening procedures, HPV testing is frequently used in conjunction with or after cervical cytology (Pap smear). When it comes to identifying the risk of cervical neoplasia, HPV tests such as PCR are more sensitive than cytology alone.
Vaccination
Why get vaccinated against HPV?
- Prevents infection with high-risk HPV types before exposure to the virus
- Lowers the risk of cervical and other HPV-related malignancies
- Vaccines are most effective when administered before the first sexual encounter (recommended 9–14 years); can also be administered to older people (some benefit up to ~45 years and beyond).
Types of vaccine
The number of HPV types that vaccinations protect against determines their classification:
Bivalent vaccine: guards against the two high-risk HPV strains (16 and 18) that cause the majority of cervical cancer cases. The primary difference between these vaccines is their coverage; higher-valency vaccines offer protection against a greater number of HPV strains.
- Quadrivalent vaccine: Prevents genital warts and cervical cancer by protecting against four HPV strains (6, 11, 16, and 18).
- Nonavalent vaccine: Provides the broadest protection against HPV-related malignancies and genital warts, protecting against nine HPV types (6, 11, 16, 18, 31, 33, 45, 52, and 58).
Available Vaccines
1. Merck’s Gardasil-9
Type: Nonavalent (protects against nine different strains of HPV: 6, 11, 16, 18, 31, 33, 45, 52, and 58)
Benefit: Provides the broadest defence against HPV types connected to genital warts and cancer. Use: Accepted in numerous nations; accessible in private healthcare settings in India Ideal for: People who can obtain comprehensive protection at private clinics. Reference – https://www.merckvaccines.com/gardasil9/
2. CERVAVAC (by Serum Institute of India)
Type: Quadrivalent (targets HPV types 6, 11, 16 & 18 – similar to Gardasil-4)
Indian-made: First indigenous HPV vaccine developed and approved in India, improving availability and affordability.
Advantage: More affordable than many imported vaccines and included in some government programs or private clinics
Best for: Widespread use through immunization programs and accessible vaccination for the general population. Reference – https://www.seruminstitute.com/product_ind_cervavac.php
Note: Kindly contact your certified physician or healthcare provider to know more about available vaccines.
Conclusion:
Early diagnosis of Human Papillomavirus (HPV) infection combined with timely vaccination plays a crucial role in the prevention of cervical cancer and other HPV-related diseases. HPV testing allows early monitoring and treatment, it can identify high-risk infections before symptoms appear or cellular alterations manifest. By avoiding infection with the most prevalent HPV forms that cause cancer, vaccination offers long-term protection, particularly when administered prior to exposure. Getting tested is crucial since HPV infections are frequently latent and can go years without being diagnosed, which raises the risk of cancer if left untreated.
Regular HPV screening and vaccination work well together to lower the burden of disease, enhance results, and save lives.

About the writer –
Ms. Aditi Sahu is an Application Specialist with over five years of experience in molecular biology and diagnostic testing, backed by an MSc in Microbiology. She began her professional journey as a frontline COVID-19 laboratory professional, supporting large-scale RNA extraction and RT-PCR testing during the pandemic. Her experience spans molecular quality control, infectious disease diagnostics, oncology and STD panels, and HPV testing. She has worked with automated platforms including Roche CAP/CTM and the Abbott m2000 system. Aditi brings a strong focus on quality, standardization, and dependable molecular diagnostic practices.
Latest Articles
How BX51WI Microscopes Support High-Resolution Neural Imaging
BY DSS Imagetech Pvt Ltd February 24, 2026
Picture this scenario. It’s 6:00 PM on a Friday. You have spent the last six hours harvesting tissue. You perfused the mouse perfectly, the liver cleared instantly, and the brain...
Read MoreThe Role of Research & Development in Driving Scientific Innovation
BY DSS Imagetech Pvt Ltd February 24, 2026
Human progress is not an accident. The leap from a simple medicinal herb to a targeted biologic drug, from a magnifying glass to a digital microscope, or from a basic...
Read MoreBeyond the Microscope: Detecting Genomic Changes with D024 KaryoProfiler
BY DSS Imagetech Pvt Ltd February 17, 2026
In the Quiet Hours of the Lab In the quiet hours of a cytogenetics laboratory, cells are busy at work while no one is watching. They divide, adapt—and sometimes, silently,...
Read More