DSS: Redefining Biotechnology & Life Science in India
Tumor Comprehensive Genomic Profiling Panel Assay
The Tumor Comprehensive Genomic Profiling (CGP) Panel Assay is a high-throughput sequencing (NGS)-based solution for the comprehensive analysis of tumor-related genes. Designed for research use only (RUO), it enables precise detection of single nucleotide variations (SNVs), small insertions/deletions (Indels), copy number variations (CNVs), and gene fusions across a 1200-gene panel. Available Test Format: 24 samples/Kit
Hereditary Cancers Panel Assay
The Hereditary Cancers Panel Assay is designed for comprehensive genetic analysis of hereditary tumor-related genes using next-generation sequencing (NGS). This assay enables accurate detection of single nucleotide variations (SNVs) and short insertions/deletions (Indels) across 58 clinically relevant genes associated with hereditary cancer syndromes. Available Test Format: 24 samples/Kit
Human Glioma 26 Gene Mutations Detection Kit (High-Throughput Sequencing)
The Human Glioma 26 Gene Mutations Detection Kit is a high-throughput sequencing (NGS)-based assay for the qualitative detection of key glioma-associated genetic alterations. Designed for research use only, the kit analyzes nucleic acids extracted from FFPE or fresh tissue samples, enabling detection of SNVs, indels, gene fusions, CNVs, and chromosome variations (1p/19q co-deletion, chr7 amplification, […]
Solid Tumors 107 Gene Mutations Detection Kit (High-Throughput Sequencing)
The Solid Tumors 107 Gene Mutations Detection Kit is designed for qualitative detection of 107 tumor-related gene mutations using high-throughput sequencing (NGS). Compatible with both Illumina and MGI sequencing platforms, this kit supports detection of SNVs, indels, gene fusions, CNVs, and MSI from FFPE or fresh tissue samples. Sequencing instruments: Illumina sequencing instruments (Miseq, NextSeq […]
Thyroid Cancer Gene Mutation Detection Kit (High-Throughput Sequencing)
The Thyroid Cancer Gene Mutation Detection Kit is a high-throughput sequencing (NGS) solution designed for the comprehensive detection of key pathogenic mutations associated with thyroid cancer. Using DNA and RNA extracted from fine needle aspiration (FNA) samples or thyroid tissue, the kit covers 16 DNA-level genes and 87 RNA-level genes (209 fusion forms). It detects […]
BRCA1/2 Gene Mutations Detection Kit (High-Throughput Sequencing)
The BRCA1/2 Gene Mutations Detection Kit is designed for the qualitative detection of BRCA1 (NM_007294) and BRCA2 (NM_000059) gene mutations in samples extracted from formalin-fixed paraffin-embedded (FFPE) tissue or peripheral blood of cancer patients. These genes are key biomarkers in hereditary breast and ovarian cancers, consisting of 23 and 27 exons respectively. The assay also […]
Oncology Multi-Gene Mutations Detection Kit (High-Throughput Sequencing)
“Oncology Multi-Gene Mutations Detection Kit (High-Throughput Sequencing) is designed for the detection of 482 somatic mutations across 13 key cancer-related genes in patients with non-small cell lung cancer and colorectal cancer. Using advanced Next Generation Sequencing (NGS) technology and RingCap®-mediated amplification, it enables precise identification of single-base mutations, insertions, deletions, and gene fusions. The kit […]
PD-L1 IHC 22C3 pharmDx
PD-L1 IHC 22C3 pharmDx Advancing Immunotherapy Options Cytotoxic T cells work to detect and eliminate infected cells and tumor cells from the body.
Antibodies & Controls
Primary Antibodies are intended for use on tissue sections whereas control Reagents are well-suited as qualitative negative controls for the primary antibodies.
PD-L1 IHC 28-8 pharmDx
PD-L1 IHC 28-8 pharmDx Advancing Immunotherapy Options Cytotoxic T cells work to detect and eliminate infected cells and tumor cells from the body.
PathVysion
The PathVysion HER-2 DNA Probe Kit II (PathVysion Kit II) is designed to detect amplification of the HER-2/neu gene via fluorescence in situ hybridization (FISH) in formalin-fixed, paraffin-embedded human breast and gastric cancer tissue specimens.
UroVysion
The UroVysion® Bladder Cancer Kit (UroVysion Kit) is FDA approved and designed to detect aneuploidy for chromosomes 3, 7, 17, and loss of the 9p21 locus via fluorescence in situ hybridization (FISH) in urine specimens from persons with hematuria suspected of having bladder cancer.
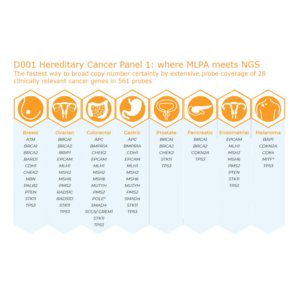
D001 Hereditary Cancer Panel
SALSA® digital Multiplex Ligation-dependent Probe Amplification (digitalMLPA) is a multiplex PCR followed by Illumina sequencing-based amplicon quantification, for the detection of copy number variations (CNV) and specific point mutations.
Entrogen REAL-TIME PCR KITS FOR SAMPLE QUALITY ASSESSMENT
EntroGen’s Sample Quality Assessment kits provide reagents, positive controls, and standards (if applicable) to detect and quantify the amplifiable DNA extracted from patient samples or adaptor-indexed libraries intended for downstream qPCR and NGS assays, respectively.
Entrogen REAL-TIME PCR KITS FOR THYROID CANCER
Thyroid Cancer Mutation Panel provides reagents for detection of point mutations in BRAF and RAS genes, as well as RET/PTC1, 3 and PAX8/PPARγ fusion genes variants.
Entrogen REAL-TIME PCR KITS FOR MELANOMA
Entrogen's kits are polymerase chain reaction (PCR)-based assays that use allele-specific primers in a multiplex reaction to identify the presence of BRAF, NRAS, and c-Kit mutations. The assays work by amplifying mutant-specific sequences in samples that contain a mixture of mutant and wild-type DNA and rely on fluorescent probes for detection. Each reaction contains primer sets and probes for detection of the mutations, as well as an endogenous control gene.
Entrogen REAL-TIME PCR KITS FOR LUNG CANCER
All kits require a real-time PCR instrument capable of detecting FAM and VIC fluorescent probes. Additionally, the Cell-Free EGFR Mutation Detection kit requires the capability to detect ROX and CY5 fluorescent probes.
All reagents required for PCR amplification/detection, as well as validated reaction controls, are included. Columns and reagents for DNA or RNA isolation are not included.
Entrogen REAL-TIME PCR KITS FOR LEUKEMIAS
EntroGen’s One-Step Detection kits provide reagents to detect and quantify the fusion gene transcripts listed below using total RNA isolated from blood or bone marrow.
Entrogen REAL-TIME PCR GENOTYPING KIT
The UGT1A1 Genotyping Kit is a polymerase chain reaction (PCR)-based assay that uses allele-specific probes to identify the most common Irinotecan polymorphic variant.
Entrogen REAL-TIME PCR KITS FOR GASTRO-INTESTINAL STROMAL TUMORS (GIST)
EntroGen’s GIST mutation screening panel is a real-time polymerase chain reaction (PCR)-based assay that uses allele-specific primers to identify the presence of somatic mutations in c-KIT and PDGFRA genes.
Real -Time PCR Kits for Colorectal Cancer
Entrogen's Mutation Analysis Kit requires a real-time thermal cycler capable of detecting FAM and VIC fluorescence signals. This test includes reagents required for the PCR amplification and signal detection, as well as validated reaction controls. Columns and reagents for DNA isolation are not included.
JAK2 V617F Mutation Detection Kit
JAK2 V617F Mutation Detection RT-PCR Kit for sensitive and specific detection of JAK2 (Janus Kinase 2) V617F allele in DNA samples with Beta Actin as an internal control.
Magnetic Bead Nucleic acid Extraction Kit
Discover the benefits of magnetic bead-based kits for nucleic acid purification. Purify high-quality nucleic acids from Biological Samples. Save time and increase consistency using the Magnetic Bead Nucleic Acid Extraction Kit
BCR ABL Quantitative RT-PCR Kit – Major
IS quantitative, one-step RT-PCR for quantifying the IS ratios of BCR-ABL Major (M-BCR) p210 transcript in CML patient samples.
BCR ABL Quantitative RT-PCR Kit – Major, Minor, Micro
Evaluates the molecular response up to 5 log reduction which is undetectable BCR-ABL1.
BCR ABL Qualitative RT-PCR Kit – Major, Minor, Micro
Single-Tube, Multiplex Real-Time PCR Assay for sensitive and specific detection of Major (M-BCR) p210, Minor (m-BCR) p190, and Micro (μ-BCR) p230 BCR-ABL transcripts.
BRCA Complete™ Expanded Panel
BRCA Complete™ Expanded Panel is a complete next generation sequencing (NGS) solution for detecting clinically relevant BRCA1 and BRCA2 mutations along with extended coverage of CHEK2, PALB2, RAD51C and TP53.
NGS Targeted Hotspot Panel (THSP)
NGS THSP stands for NGS Targeted Hotspot Panel is supposed to detect the multiple clinically related hotspot mutations in 16 genes across several tumour types. Its comprehensive multi-gene panels identify hundreds of genes and detect thousands of variants, many of which have unknown factors.
Melanoma panel
It is estimated that 10% of melanoma cases are attributed to hereditary predisposition.
Hepatocarcinoma Panel
Hepatoma is called hepatocellular carcinoma (HCC) and hepatocarcinoma, it is the most common kind of primary liver malignancy.
Breast Panel
The Breast Cancer Comprehensive Panel analyzes 20 genes related to an expanded risk of hereditary breast cancer.
Neuropathology panels
Neuropathology panels focus and evaluate all aspects of neurodegeneration.
Testimonials & Reviews